In endodontic practice, the reliability and quality of instruments directly impact treatment outcomes and patient safety. MANI Files have earned global recognition for their precision engineering, consistency, and adherence to stringent quality standards. However, the increasing circulation of counterfeit products poses a significant threat to clinical performance, patient trust, and practitioner credibility. To safeguard your practice, it is essential to distinguish authentic MANI Files from counterfeit imitations. Below is a professional reference guide.
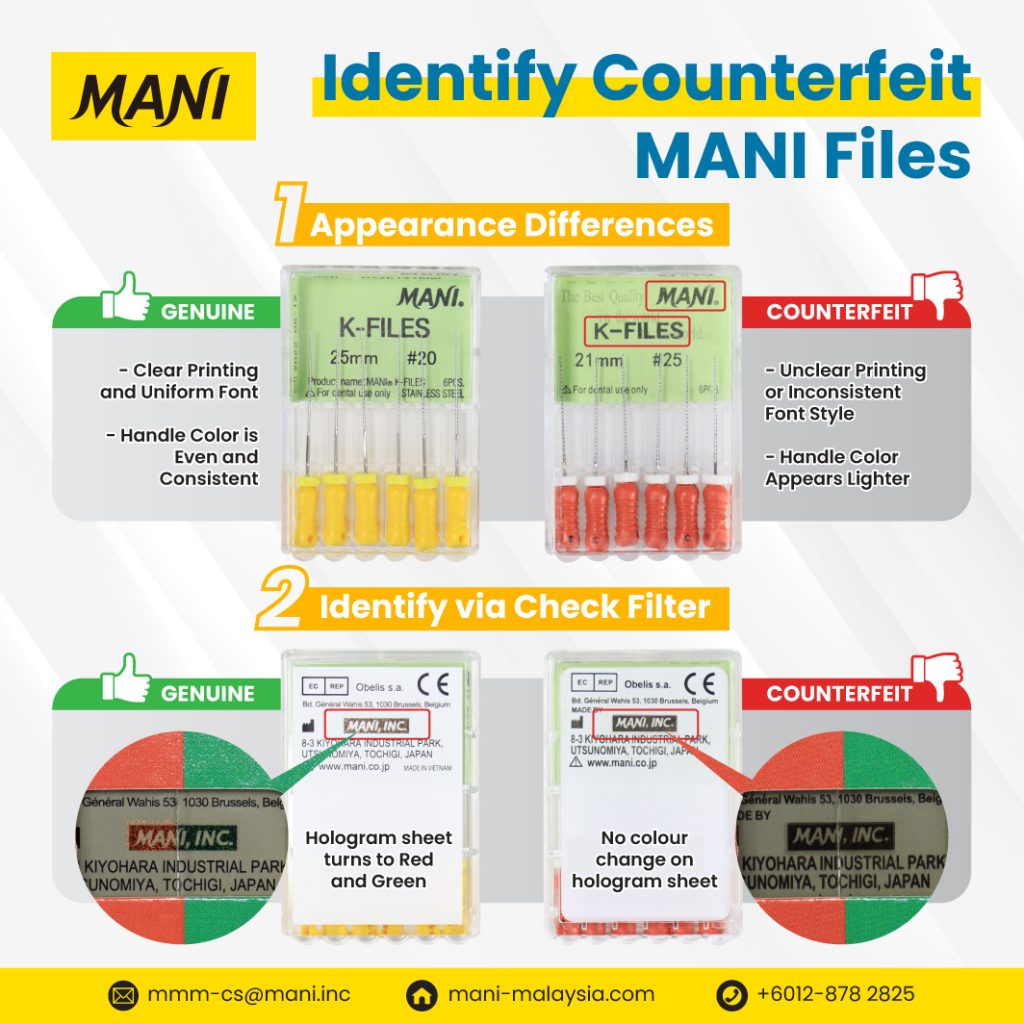
1. Distinguishing Counterfeit by Appearance
Careful inspection of packaging and instrument design is the first line of defense against counterfeit products.
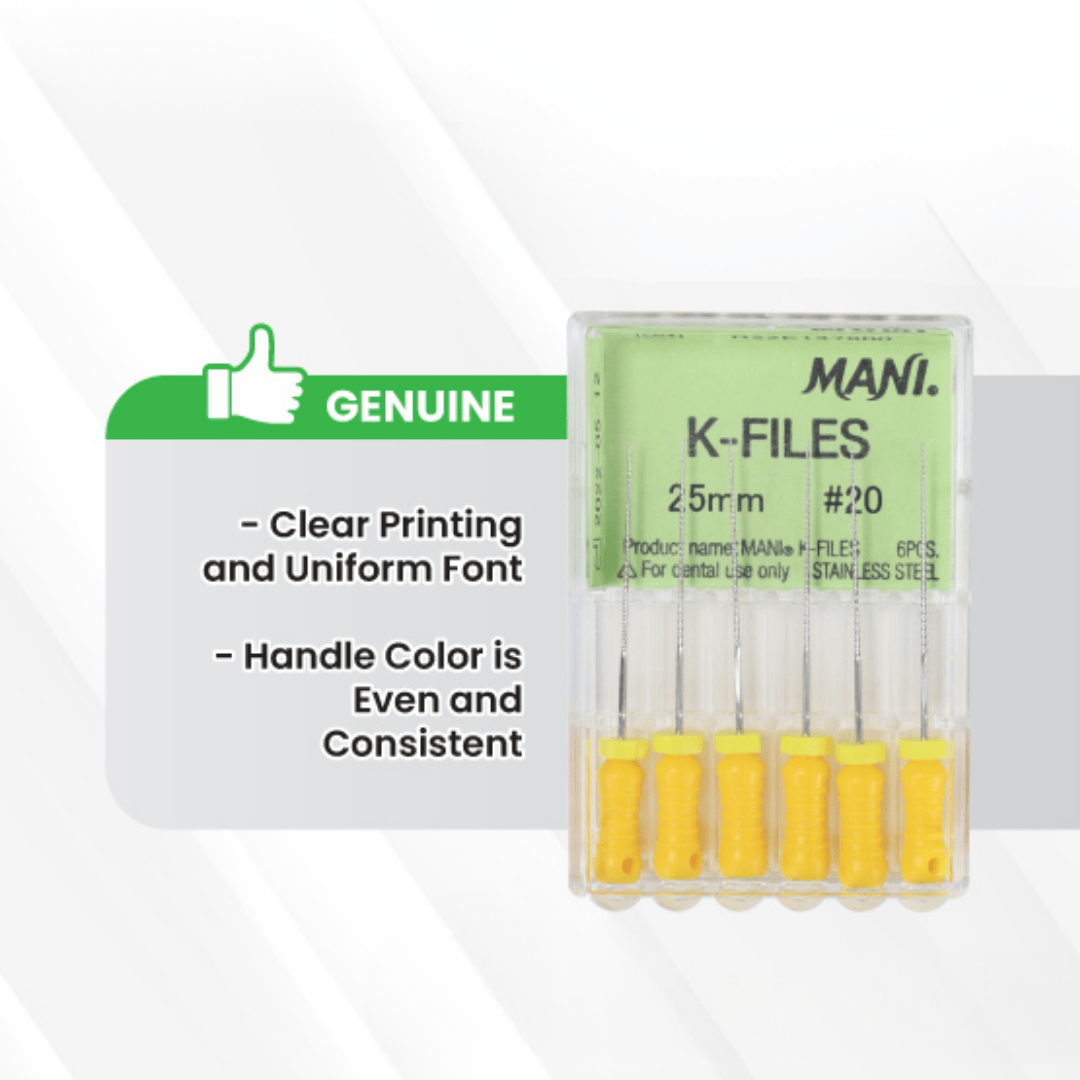
Authentic MANI Files:
Exhibit sharp, clear printing with a uniform and standardized font style.
Handle colors are vibrant, evenly applied, and consistent across the set.
Packaging demonstrates high manufacturing quality with no irregularities.
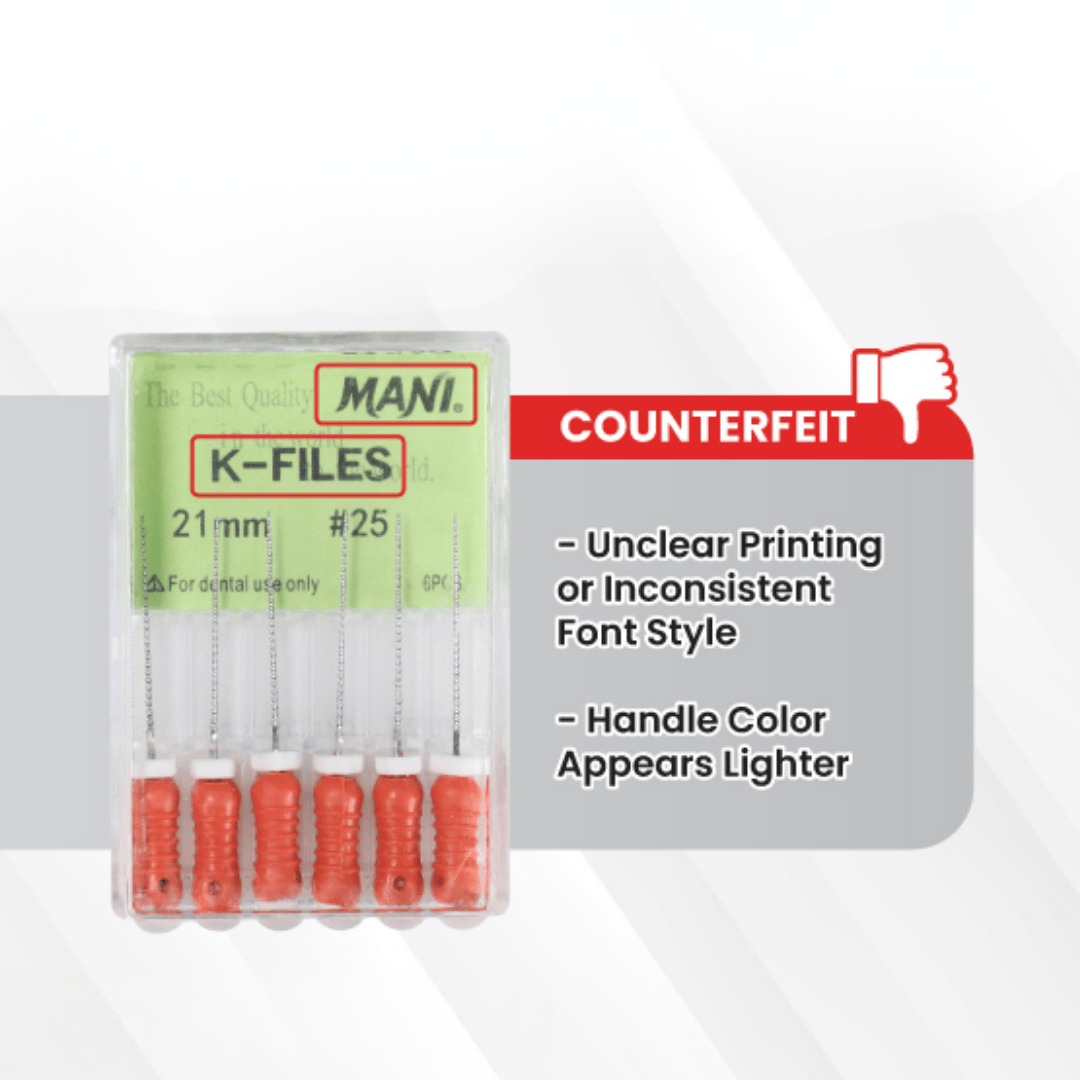
Counterfeit MANI Files:
Printing is often blurred, faint, or inconsistent in font design.
Handle coloration is lighter, uneven, or appears faded.
Packaging may display irregularities or substandard finishing.
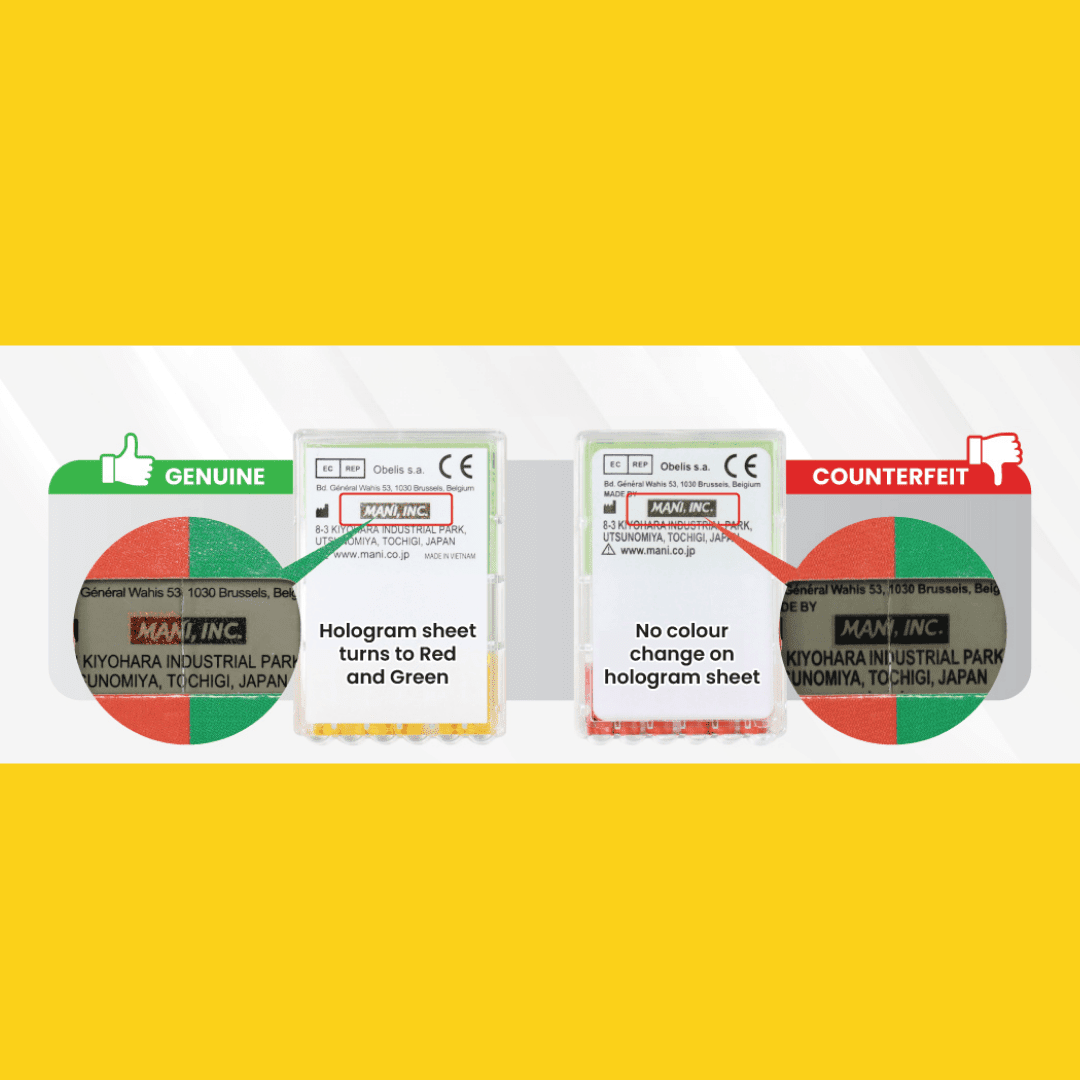
2. Verification via Hologram Security Feature
Genuine MANI products are equipped with a holographic authentication system to ensure traceability and verification.
Authentic MANI Files:
The hologram label demonstrates a distinct optical color shift, turning red and green when observed through the check filter.
This feature is consistent and reproducible, ensuring authenticity.
Counterfeit MANI Files:
Hologram labels fail to demonstrate any optical color change, remaining static under inspection.
Lack of holographic responsiveness is a clear indicator of counterfeit origin.
Clinical Significance of Identifying Counterfeit Products
Counterfeit endodontic instruments lack the rigorous quality control, material integrity, and performance standards of authentic MANI products. Their use may result in:
Increased risk of file separation and instrument failure.
Inconsistent cutting efficiency, compromising canal shaping.
Potential cross-contamination and safety risks due to uncontrolled manufacturing processes.
As professionals entrusted with patient care, it is imperative to mitigate these risks by ensuring all instruments are genuine.
Best Practices for Procurement and Verification
Purchase exclusively from authorized distributors and trusted supply partners.
Inspect packaging, print quality, and handle coloration for uniformity.
Verify hologram authenticity using the designated check filter.
Report any suspected counterfeit products to the appropriate channel for investigation.
Contact Information
For verification support, product inquiries, or to report counterfeit findings, please contact:
Email: mmm-cs@mani.inc
Website: mani-malaysia.com
Phone: +6012-878 2825
Maintaining high standards of care requires vigilance against counterfeit instruments. By familiarizing yourself with these identification protocols, you safeguard both your clinical outcomes and patient safety. Always insist on genuine MANI Files—precision-engineered for trust, reliability, and excellence in endodontic practice.